 |
|
|
|
|

Head of Regional Office
New Delhi
India is a country where the burden of neglected diseases represents major health problems. It is also a country where one can find a talent pool of research and science experts with the added advantage of cost-effectiveness. As a response, and with support from the India Council of Medical Research (ICMR), DNDi opened the Regional Support Office in India in 2007 to work as relay to DNDi's operational activities, namely in the field of two diseases, malaria and visceral leishmaniasis. According to the Indian Ministry of Health, these two diseases alone affect 3 million Indian citizens each year.
Two discovery projects on HAT and VL
Collaboration in screening for new and existing chemical compounds has been established with Central Drug Research Institute, Lucknow for human African trypanosomiasis (HAT). The study is for lead identification among the existing chemical classes.
A VL consortium was also established with Indian institutions for lead optimisation of compounds for VL. Advinus in Bangalore has a team of dedicated chemists who are synthesising compounds which are then screened at CDRI Lucknow or LTSTMH (UK) for in vitro and in vivo activities. If the results prove satisfactory following lead optimisation, the compounds will be taken forward for preclinical studies and consequent clinical evaluation.
Malaria Clinical Trial Studies
Malaria is unevenly distributed in India as 80% of the population lives in low transmission areas whereas 20% in a high transmission belt. The annual burden reported by the national malaria control programme is of 2 million confirmed cases and 1000 deaths, whereas the WHO estimates 15 million cases and 20,000 deaths. India holds 77% of the Southeast Asia malaria burden.
DNDi's answer through the FACT Projects: ASAQ and ASMQ have been recommended for implementation in India in areas where there exist multiple drug resistance. For this purpose, clinical trials are put in place for the two combinations in order to obtain clinical evidence on their efficiency and safety. Clinical trial study on ASAQ was developed with the National Institute of Malaria Research (NIMR) in India in order to evaluate the ASAQ FDC and AQ in the malaria endemic regions of Orissa and Jharkhand. The results of the study are very promising. ASMQ is also currently being evaluated with NIMR in the Goa and Assam regions. A partnership has been established with a private pharmaceutical industry and the technology will be transferred from the public pharmaceutical firm Farmanguinhos/Fiocruz in Brazil for the production of ASMQ.
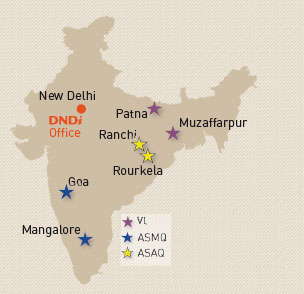
VL Combination Study
India has about 100,000 new cases of VL annually of which approximately 90 per cent are from Bihar. Up to recently, Pentavalent antimony complex was one of very few standard treatment of VL with all its limitations: toxicity, lengthy treatment, and growing resistance. Recently, amphotericin B, lipid formulations of amphotericin B, paromomycin, and miltefosine have been evaluated and approved for the treatment of VL. All of these drugs have advantages and disadvantages with regards to cost, time, toxicity, and administration ease. Therefore, to reduce the time course of therapy, to increase compliance, and to decrease resistance, development of combinations of these drugs in VL has been proposed. A Clinical Study to evaluate various combinations has been initiated in collaboration with RMRI Patna and KMRC Muzaffarpur. The study will furthermore be extended to Nepal and Bangladesh, for recommended use in the national programmes for the highly endemic regions of VL.