 |
|
|
|
|

Synergise Efforts
|
Anacor’s Boron-Based Compounds to be Used Against Kinetoplastids
Anacor Pharmaceuticals, a biotech company based in Palo Alto, and DNDi have signed a collaboration agreement whereby DNDi has gained access to Anacor's proprietary boron-based chemistry for use against selected targets of parasites causing neglected diseases like visceral leishmaniasis (VL or kala azar), human African trypanosomiasis (HAT or sleeping sickness), and Chagas disease.
Anacor's proprietary technological advances in the synthesis of boron-based compounds coupled with its rational drug design expertise, has enabled the company to rapidly create diverse families of boron-based compounds, some of them with promising against kinetoplastids. The collaboration will utilize Anacor’s propietary chemical series, shown to be active in vitro against the various parasites, to optimise and select new molecules with in vivo activity and to further develop them. DNDi will then be responsible for development, including selecting lead candidates, conducting clinical studies, and, ultimately, distributing the new medicines. "We look forward to a successful partnership with DNDi as we strongly believe in the potential of our boron-based chemistry to contribute to improved international health”, remarked Anacor’s CEO David P. Perry. “This agreement with DNDi provides an innovative approach for developing truly novel medicines to treat the most neglected diseases and for combating problems of resistance."  ©Anacor's Boron-Based Compound Agreement with IRD on Two Molecule Groups as Potentials for Drug Candidates
The public Paris-based Institut de Recherche pour le Développement (IRD) and DNDi join forces in carrying out scientific cooperation projects for the development of treatments against Chagas disease, visceral leishmaniasis, and sleeping sickness. One of these projects linked to canthin-6-one compounds has entered the optimisation phase which is a step forward towards becoming a drug candidate.
Each party signed two collaboration agreements aimed at identifying and developing promising new drug candidates against visceral leishmaniasis which will lead to optimisation of compounds of quinolines for VL and canthin-6-one for Chagas, whose characteristics are showing potential therapeutic efficacy. This type of agreement will allow DNDi to enrich its R&D project portfolio. These two molecules were respectively patented by both IRD and CNRS in 2001 (1) and IRD and one of its southern partners, National University of Asunción, Paraguay, in 2003 (2).  Leishmania Promastigote, ©IRD/Christian Bellec Cooperative Agreement between UNC and DNDi to Facilitate R&D Innovation
The University of North Carolina (UNC) and DNDi have entered into a synergistic collaboration with each other so as to ensure that needs-driven R&D for the most neglected diseases is handled in the most efficient manner possible. Through a cooperative agreement, DNDi and CPDD will work to prevent any duplication of research efforts, to identify projects of common interest where collaboration could result in improved product development efficiency, and to provide independent review, in-depth analysis, and information sharing between the two institutions so as to avoid any redundant efforts and unnecessary spending for the development of new anti-parasitic treatments. “This collaboration marks an excellent opportunity to strengthen both parties’ efforts, achieve significant international benefits in their own fields of drug research, and to align strategies that will ultimately facilitate project management and patient access to innovation,” remarked Dr. Richard R. Tidwell, Professor of Pathology and Laboratory Medicine at the UNC School of Medicine and Director of the UNC-led Consortium for Parasitic Drug Development (CPDD).
|
A Collaborative Research Effort with GSK
"We hope this and similar alliances serve as a model for future drug development for neglected diseases," said Dr. Federico M Gomez de las Heras, Vice President of the Infectious Diseases Center of Excellence in Drug Discovery (ID CEDD) at GSK in March 2008 when a collaborative research effort was announced targeting neglected tropical diseases which disproportionately affect the developing world. This research programme will focus on compounds that may be active against the most neglected diseases of visceral leishmaniasis (kala azar), human African trypanosomiasis (sleeping sickness), and Chagas disease. Collaboration, which has been established for an initial period of two years and may be extended, will focus on identifying and developing compounds from existing GSK programmes and will leverage the expertise of researchers from GSK at its Tres Cantos (Spain) facility, a dedicated drug discovery unit within GSK R&D organisation, to maximise efforts in this area, along with leading academic centres like the London School of Hygiene & Tropical Medicine (LSHTM).
Eskitis, MMV, and DNDi Share Information and Harmonise their Research Activities
The Eskitis Institute in Brisbane, Australia has a chemical library of around 300,000 natural products derived from plants in Australia, Papua New Guinea, and China, as well as from Australian marine invertebrates. The Natural Product Discovery Programme was originally established in 1988 between pharmaceutical giant AstraZeneca and Griffith University and is one of the most successful public-private partnerships in Australia. AstraZeneca committed USD 100 million to the programme up to the end of 2007. Eskitis has now made its library available for screening against neglected diseases and both DNDi and the Medicines for Malaria Venture (MMV) have entered into research agreements with Eskitis to screen the library against parasites responsible for sleeping sickness and malaria. To guarantee the maximum benefit, all three partners work together to share information and harmonise the research.
 ©Eskitis Institute Pasteur Korea: When High-Tech Meets Neglected Diseases
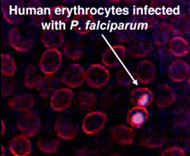
DNDi initiated a partnership with the Institut Pasteur Korea (IP-K) with the goal to identify active compounds against Leishmania spp., the parasite that causes leishmaniasis, a neglected disease that affects over 12 million people and puts 350 million others in 88 countries at risk. With its unique infrastructure (Evotec-Opera system housed in a BSL-3 laboratory) and advanced and automated confocal microscopy technology in collaboration with imaging tools, IP-K is developing and validating a high-throughput visual screen to be used in screening compounds against the clinically relevant form of Leishmania spp. This will include the adaptation of Leishmania donovani intramacrophagic amastigote culture to the HTS screening platform as well as the required algorithms for image analysis (optimisation of the software for the confocal readouts and computational analysis of the pictures) before the screening of a 200,000-compound library will be carried out.
When successful, this project will represent a major breakthrough in the development of drugs for the most neglected diseases as it will be the first HTS assay against the clinically relevant intracellular (amastigote) stage of Leishmania parasites. Moreover, this technology could then be extended to other intracellular parasites such as T. cruzi, the etiological agent of Chagas disease.  The Evoscreen Platform for Visual Screening, IPK - (Photo taken from www.pasteur.or.kr) |
Published by Drugs for Neglected Diseases Initiative - 15 Chemin Louis-Dunant 1202 Geneva Switzerland - Photo credits: DNDi unless otherwise stated - Editor: Sadia Kaenzig - Tel: +41 22 906 9230 - Fax: +41 22 906 9231 - www.dndi.org