 |
|
|
|
|




The Indian subcontinent (India, Nepal & Bangladesh) is one of the main areas affected by VL worldwide. It accounts for about 67% of total cases reported(1) with almost 200 million people at risk of contracting the disease(2). The governments of India, Bangladesh and Nepal have launched a joint programme to eliminate VL as a public health problem by the year 2012. Recent advances in the area of VL treatment such as the introduction of the first orally administered drug, miltefosine, the milestone introduction of an affordable drug, paromomycin (by Institute of One World Health – iOWH - with funds from the Bill and Melinda Gates Foundation, BMGF) and WHO negotiated preferential price for the safe and efficacious drug, liposomal amphotericin B (AmBisome®), would appear to bode well for elimination of VL in the region.
Combination therapy: the shorter the better
To address the current drawbacks of available treatments in India, the overall objective of the VL combination study run by DNDi and its partners is to reduce the duration of treatment, currently between 21 and 30 days, for monotherapy, to a maximum of 11 days for combination treatment. This would shorten hospitalisation time; reduce the need for patient-health worker contact thereby lessening the burden on patients and the health system; reduce the chance of emergence of resistant organisms to single drugs; and potentially decrease toxicity through the reduced duration of the treatment and dose of drugs. The use of combination therapy will reduce the cost of patient care and the financial burden to patients associated with prolonged displacement and high cost of treatment.
With the introduction of new therapies, including miltefosine in 2002 and paromomycin in 2006, it is essential that there is a strategy in place to prevent the emergence of resistance to new drugs - combination therapy, monitoring of therapy, and improved diagnostics could play an essential role in this strategy.(3)

“Over the next few years, efficacy and safety results from several trials of combination therapies will become available, and we will hopefully identify a number of safe and effective options. In determining which combination therapy to select within a given context, we must remember certain factors like regional differences in drug susceptibility, local health infrastructure and capacity to treat, and cost effectiveness”, said Dr Johan Van Griensven, of the Institute of Tropical Medicine in Antwerp, Belgium.
(2) Joshi A, et al. Can visceral leishmaniasis be eliminated from Asia? J.Vector Borne Dis. 2008;45:105-111.
(3) Sundar, et al.; Clinical Microbiology Reviews, January 2006, p. 111-126, Vol. 19, No. 1.
A definitive phase III combination therapy trial has been designed to provide data for authorities in India, Bangladesh and Nepal to make informed recommendations for combination treatment which can be used in the elimination programme. This is a multicentre trial in two sites in Bihar, India - one at Kala-Azar Medical Research Centre, Muzaffarpur, and the other at Rajendra Memorial Research Institute of Medical Sciences, RMRIMS (ICMR), in Patna. This trial started in June 2008 in both sites with the enrolment of adults (118 year-old patients). Based on evaluation of the response of 120 patients in different treatments, approval from the Drug Controller General of India (DCGI) has been obtained and children over the age of 5 are being enrolled. So far, 465 patients out of a total 624 have been enrolled. The trial is expected to be completed by the end of this year and results should be available early in 2010.
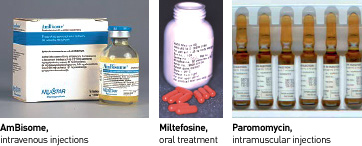
This study is a randomized, open-label, parallel group, non-inferiority (17%) study to compare 3 combination treatment regimens to standard treatment for VL in India. In fact, the National Drug Policy of India currently recommends first-line treatment with amphotericin B deoxycholate which is administered as an IV infusion on alternate days over 30 days (in hospital for close observation of side effects) and miltefosine, administered as an oral tablet once or twice daily for 28 days in certain VL endemic areas as part of the national elimination programme. Miltefosine should only be administered to women of child-bearing age who are using contraception due to its potential teratogenicity. The safe and efficacious drug AmBisome®, developed more than a decade ago, remains unaffordable (US280 for 35kg adult) if used at the full dose of 20mg/kg, even at the price negotiated with WHO.
The treatment regimens of the phase III combination therapy trial are:
- AmBisome® 5mg/kg IV (intravenous) infusion (single dose) followed by oral miltefosine 2.5 mg/kg/day for 7 days
- AmBisome® 5mg/kg IV infusion (single dose) followed by paromomycin sulphate 15mg/kg/day i.m (intramuscular injection) for 10 days, and
- oral miltefosine 2.5mg/kg/day + paromomycin sulphate 15mg/kg/day i.m. for 10 days
(1) Joshi A., et al., Can Visceral Leishmaniasis be eliminated from Asia?
J. Vector Borne Dis. 2008; 45: 105-111
 “From July 2007 to December 2008, we have been studying the safety and effectiveness of liposomal amphotericin B (AmBisome®, 20 mg/kg) treatment under routine programme conditions of four infusions over a period of ten days. With initial cure rates of over 98%, death rate of o0.5%, and high tolerability seen in a total of 2.538 patients, we are very encouraged by these results and believe that further combination studies with AmBisome® as the main drug are a priority. We look forward to seeing our working collaboration with DNDi in Bihar grow.” commented Dr Nines Lima, from MSF-Spain who has served as an advisor in the MSF-supported visceral leishmaniasis treatment programme in Bihar.
“From July 2007 to December 2008, we have been studying the safety and effectiveness of liposomal amphotericin B (AmBisome®, 20 mg/kg) treatment under routine programme conditions of four infusions over a period of ten days. With initial cure rates of over 98%, death rate of o0.5%, and high tolerability seen in a total of 2.538 patients, we are very encouraged by these results and believe that further combination studies with AmBisome® as the main drug are a priority. We look forward to seeing our working collaboration with DNDi in Bihar grow.” commented Dr Nines Lima, from MSF-Spain who has served as an advisor in the MSF-supported visceral leishmaniasis treatment programme in Bihar.