 |
|
|
|
|

Developing New Health Tools
for VL Patients
for VL Patients
Interview by Sadia Kaenzig
Media and Corporate Communications Manager
Media and Corporate Communications Manager
Therapeutic research linked to visceral leishmaniasis (VL) is complex and limited, because it has been a fully neglected disease. Dr Shing Chang, DNDi ’s R&D Director, explains how DNDi is addressing the R&D challenges posed by VL.

DNDi actively participated in the recent Fourth World Congress on Leishmania in India. How much of a challenge is VL today?
VL, also called Kala-azar or black fever, persists today in poor, remote, and sometimes politically unstable areas, and claims around 51,000 lives every year. The majority of the 500,000 new cases each year affect children and it is estimated that only 30% of cases are reported. So VL is a serious challenge for public health.
Everybody agrees that chemotherapy remains one of the most important tools in the control of VL. We still face several barriers that prevent the world’s poorest from getting the drugs, for example: lack of access to functional treatment centres; high treatment costs; poor drug efficacy and tolerability; and long treatment duration. DNDi aims to address these needs by developing safe and efficacious treatments which are field adapted and which could delay the emergence of drug resistance.
What does DNDi do to address these challenges?
Significant work has already been undertaken in the field of VL drug development. A few new treatments have been developed recently, but these have limitations in monotherapy. Very simply, our short-term strategy is based on better use of existing treatments in combination therapy; in the long term, the focus is on development of new drugs. By 2014, we expect to deliver five new VL treatments, specifically: two geographical extensions; two new combination therapies and have one new drug registered. During this timeframe, we also aim to advance at least one new candidate to phase II/III clinical development.

How do you expand treatment options and develop improved treatments for patients?
As I mentioned earlier, there are few VL drugs available. One is AmBisome™, an amphotericin B liposome formulation, registered for VL in the USA and Europe. Another is oral miltefosine, registered in India in 2002, now in use in monotherapy. A low cost parenteral (intramuscular) formulation of paromomycin (aminosidine) was registered in India after a study developed by our colleagues from iOWH. But because appropriate clinical studies supporting their use in some endemic countries have not been carried out, they are not approved in all endemic countries. Where they are available, they are used as stand-alone treatment (monotherapy). To increase treatment options, DNDi has opted for geographical extension programmes of existing drugs by providing necessary data to support their registration in these endemic countries that have yet to approve them. However, we realise that there are differences in drug effectiveness from one region to the other and this is clearly one of the challenges we have been confronted with over the past years. For instance, in our attempt to register paromomycin in East Africa, early results of our clinical studies showed in 2006 that the initial dosage of the drug did not work as well in Africa as it did in India - this is why we are now testing a higher dosage of the drug in the hope that it may work better in Africa.
Click on thumbnail below to view larger picture:
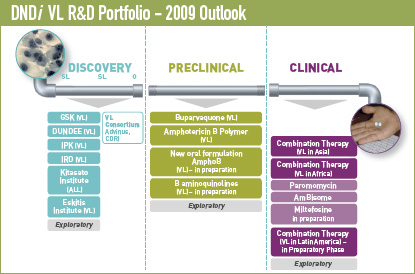
The development of innovative drug combinations is a key part of our strategy, which could offer important advantages such as: better tolerability; a lower burden on the health systems in resource-limited areas; shorter courses of treatment; cost effective treatment; and most importantly, reduced resistance development.
When two drugs are used in combination, the risk of developing resistance is greatly reduced. The big advantage of this is that it prolongs the useful life spans of the few existing drugs. As a matter of fact, all ongoing DNDi clinical trials carried out in collaboration with different partners fall under the category of developing improved treatments with currently available tools.
What about the future? How do you intend to develop new drugs which are more efficacious and simple to use in remote settings?
New drugs come from the long process of compound screening and lead optimisation. These require access to a wide range of different compounds from which one hopes to identify a few with selective activity against leishmania parasites. To achieve this, we have established partnerships with academic and industrial institutions including Basilea, Novartis Institute for Tropical Diseases (NITD), GlaxoSmithKline (GSK), Advinus, Central Drug Research Institute (CDRI), Anacor, Institut Pasteur Korea (IPK), and others.
As it can take 10 years to bring a compound through the preclinical and clinical phases of development, DNDi also aims to accelerate the development and registration of new VL drugs by seeking compounds that have already shown some promising preclinical and early clinical data on safety and efficacy. We are currently considering several potential products such as buparvaquone, a new amphotericin B formulation, and aminoquinolines that could be made available to patients as early as 2014. This will occur only if clinical benefits for the patients are demonstrated in the clinical studies that we intend to carry out.
How does DNDi bring together partners for a sustainable R&D innovation?
We certainly cannot do it all on our own. Developing drugs for VL is a long and arduous process that requires skilled and knowledgeable partners throughout all stages of the pipeline and into the field. DNDi follows a virtual research model whereby most research is outsourced and actively managed by DNDi research project managers experienced in pharmaceutical development. We also have to engage in partnership with national control programmes and specialists in the field early in the process, for their disease-specific knowledge and networks. The Leishmaniasis East Africa Platform (LEAP), for instance, is well placed to gather data, strengthen capacity, and conduct effective clinical trials in East Africa. DNDi also provides support to develop the capacity of national health authorities when appropriate. Obviously, all these early and strong partnerships with implementers should facilitate future access to new tools.
Importantly, we need to promote synergies and secure enhanced, sustainable funding to develop and to provide accessible treatments for VL patients who are neglected. We know what it takes to beat VL and we need everyone’s support to make it happen.
What is DNDi ’s VL Strategy to address unmet treatment needs?
DNDi ’s VL-specific portfolio balances short- and long-term objectives.
Short term: better use of existing treatments through geographical extension and new combinations. To deliver new treatments by 2014 through combination and/or geographical extension of existing treatments through two geographical extensions and two coadministrations.- Combination of existing therapies for VL in Africa:
Registration of Paromomycin in 2010
Recommendation of Paromomycin + SSG Combination
Registration of AmBisome in 2011 (geographical extension) - Combination of existing therapies for VL in India:
Recommendation in India, Bangladesh and Nepal by 2011 - Combination treatment in Latin America:
Recommendation of combination in 2013
Long term: To register one new drug through new formulations of existing treatments and therapeutic switching, and to have one new candidate in Phase II/III clinical development.
- 8-aminoquinolines – currently GlaxoSmithKline (GSK) has two potential preclinical candidates (Tafenoquine and Sitamaquine) for development.
- New formulations of amphotericin B – BioDelivery Sciences International (BDSI) has developed an oral formulation that is currently in Phase I, targeting fungal infections. DNDi is conducting a preclinical evaluation of this oral formulation for VL and, if successful, will proceed with clinical development.
- Backup compounds in-sourced at late preclinical phase – DNDi is actively pursuing potential candidates that could be ready for clinical development in the short term.
Published by Drugs for Neglected Diseases Initiative - 15 Chemin Louis-Dunant 1202 Geneva Switzerland - Photo credits: DNDi unless otherwise stated - Editor: Sadia Kaenzig - Tel: +41 22 906 9230 - Fax: +41 22 906 9231 - www.dndi.org