 |
|

NECT: Next Steps
In order to facilitate the availability of NECT, DNDi and partners will continue to build the evidence base for the use of NECT as a treatment for stage 2 HAT. The following are three key action items.
Submitting an application to the WHO Essential Medicines List:
Work is well advanced into drafting an application for the inclusion of nifurtimox, to be used in combination with eflornithine, into WHO’s 2009 Essential Medicines List (EML) for stage 2 T. b. gambiense HAT.
• Both treatments are already on the list as essential medicines, but nifurtimox is listed only for the treatment of Chagas disease. The application will be strictly for its use in combination therapy to treat stage 2 T. b. gambiense HAT
• Basis of application include the final results of the NECT study as well as a systematic review of available stage 2 HAT treatments
• Those who are interested can review the application online. The EML Committee that reviews and approves applications will meet end of March 2009. To learn more about the EML process, visit the WHO Essential Medicines website: http://www.who.int
NECT-FIELD Studies: Evaluating the tolerability, feasibility, and effectiveness of NECT in “real-life” conditions:
This pragmatic trial will document the use of NECT as a first-line treatment for stage 2 in ‘ordinary’ HAT treatment centres, and can pave the way towards a broader adoption of this treatment.
• Partners for this study include PNLTHA, DRC; SCIH-STI; MSF-CH
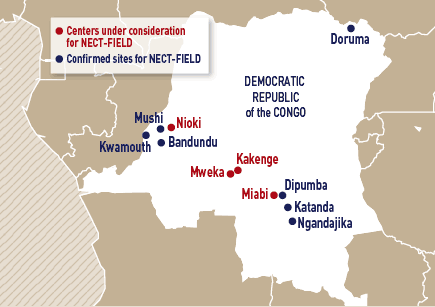
• Study is expected to begin by early 2009 with the following sites (see map): Dipumba, Katanda, and Ngandajika in East Kasai; Bandundu, Mushi, and Kwamouth in Bandundu Province, and Doruma in Equatorial province. Further sites are under consideration
Establishing an appropriate dose regiment for use of NECT in children:
Consensus exists that studies are needed in order to establish the appropriate paediatric dose regimen forNECT.
• Convening in October 2008, an expert committee will advise on the best strategy for identifying and validating an appropriate dose for NECT in children as paediatric patients are a substantial portion of the population
• Based on the outcome of the expert consultation, the NECT-FIELD study may be amended to include children of younger age groups; otherwise, an alternative study will designed so as to adequately address the issue.
Submitting an application to the WHO Essential Medicines List:
Work is well advanced into drafting an application for the inclusion of nifurtimox, to be used in combination with eflornithine, into WHO’s 2009 Essential Medicines List (EML) for stage 2 T. b. gambiense HAT.
• Both treatments are already on the list as essential medicines, but nifurtimox is listed only for the treatment of Chagas disease. The application will be strictly for its use in combination therapy to treat stage 2 T. b. gambiense HAT
• Basis of application include the final results of the NECT study as well as a systematic review of available stage 2 HAT treatments
• Those who are interested can review the application online. The EML Committee that reviews and approves applications will meet end of March 2009. To learn more about the EML process, visit the WHO Essential Medicines website: http://www.who.int
NECT-FIELD Studies: Evaluating the tolerability, feasibility, and effectiveness of NECT in “real-life” conditions:
This pragmatic trial will document the use of NECT as a first-line treatment for stage 2 in ‘ordinary’ HAT treatment centres, and can pave the way towards a broader adoption of this treatment.
• Partners for this study include PNLTHA, DRC; SCIH-STI; MSF-CH
• Study is expected to begin by early 2009 with the following sites (see map): Dipumba, Katanda, and Ngandajika in East Kasai; Bandundu, Mushi, and Kwamouth in Bandundu Province, and Doruma in Equatorial province. Further sites are under consideration
Establishing an appropriate dose regiment for use of NECT in children:
Consensus exists that studies are needed in order to establish the appropriate paediatric dose regimen forNECT.
• Convening in October 2008, an expert committee will advise on the best strategy for identifying and validating an appropriate dose for NECT in children as paediatric patients are a substantial portion of the population
• Based on the outcome of the expert consultation, the NECT-FIELD study may be amended to include children of younger age groups; otherwise, an alternative study will designed so as to adequately address the issue.

Published by Drugs for Neglected Diseases Initiative - 15 Chemin Louis-Dunant 1202 Geneva Switzerland - Photo credits: DNDi unless otherwise stated - Editor: Sadia Kaenzig - Tel: +41 22 906 9230 - Fax: +41 22 906 9231 - www.DNDi.org