 |
|

Fexinidazole Progresses into Clinical Development
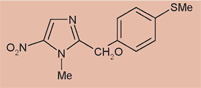 In early 2009, DNDi plans to advance fexinidazole into first-in-human, Phase I studies. Fexinidazole is the first compound to be taken by DNDi from discovery, through preclinical development, and now into clinical development. There is still much work to be done before fexinidazole might prove to be a new treatment for stage 2 HAT, but the current progress certainly raises hopes.
In early 2009, DNDi plans to advance fexinidazole into first-in-human, Phase I studies. Fexinidazole is the first compound to be taken by DNDi from discovery, through preclinical development, and now into clinical development. There is still much work to be done before fexinidazole might prove to be a new treatment for stage 2 HAT, but the current progress certainly raises hopes. A 5-nitroimidazole, fexinidazole is orally active against both T.b. gambiense and T.b. rhodesiense. It has also shown an excellent safety profile in animal studies and passes the blood-brain barrier, which means that it could be effective against both stages of sleeping sickness.
A 5-nitroimidazole, fexinidazole is orally active against both T.b. gambiense and T.b. rhodesiense. It has also shown an excellent safety profile in animal studies and passes the blood-brain barrier, which means that it could be effective against both stages of sleeping sickness. This project is the result of a unique approach taken by DNDi to build upon existing knowledge: in 2005, an extensive compound mining effort was begun to explore new and old nitroimidazoles as drug leads against sleeping sickness. This project, along with the FACT Project, was highlighted as one of DNDi ’s success stories at the recent Stakeholder’s meeting in New York, where short films on the projects were shown.
To read more about the meeting and to see the short films, please visit:
www.DNDina.org/stakeholder.
Published by Drugs for Neglected Diseases Initiative - 15 Chemin Louis-Dunant 1202 Geneva Switzerland - Photo credits: DNDi unless otherwise stated - Editor: Sadia Kaenzig - Tel: +41 22 906 9230 - Fax: +41 22 906 9231 - www.DNDi.org