 | |


HAT platform: improving
the clinical research capacity
the clinical research capacity
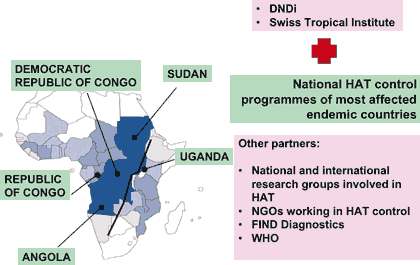
There is limited clinical research activity to assess and/or improve treatments and diagnostics for HAT, in part due to the remoteness and spread of the patients. The national control programmes of the 5 most affected countries, in collaboration with DNDi, the Swiss Tropical Institute (STI), and a number of other partners, have established a platform for capacity building in clinical trials for HAT. The overall aim of this platform is to build and strengthen clinical trial capacities in HAT disease endemic countries so that new and promising interventions for this fatal disease can be rapidly and effectively evaluated, registered and made available to the patients.
The short-term objective is to ensure that already planned and initiated clinical trials are completed successfully (DB-289, Nifurtimox + Eflornithine).
The longer term objective is to facilitate the design and implementation of new trials needed to ultimately make available new tools for the control and treatment of sleeping sickness, in particular:
1. New diagnostic tools for:
a. simple and sensitive case detection
b. accurate disease staging
c. monitoring disease progression and cure
2. Effective, safe and easy to use treatments, preferably active in both Stage 1 AND 2 disease
This platform can also provide a base for ongoing educational cooperation between the countries in the central African region, facilitating sustainable disease control activities and standardising procedures and practices.
DNDi has facilitated the creation and maintenance of this platform both financially and through general administrative and logistic support through the DNDiAfrica office in Nairobi, and the STI-DNDiAfrica representation in Kinshasa. The HAT Platform encompasses over 30 clinicians and scientists from Uganda, DRC, Republic of Congo, Sudan, Angola, DNDi, WHO, Epicentre, STI, Institute of Tropical Medicine Antwerp, Medecins Sans Frontieres (MSF), Malteser, Kari Trypanosomiasis Research Centre, Find Diagnostics, and Centers for Disease Control and Prevention (CDC).

The short-term objective is to ensure that already planned and initiated clinical trials are completed successfully (DB-289, Nifurtimox + Eflornithine).
The longer term objective is to facilitate the design and implementation of new trials needed to ultimately make available new tools for the control and treatment of sleeping sickness, in particular:
1. New diagnostic tools for:
a. simple and sensitive case detection
b. accurate disease staging
c. monitoring disease progression and cure
2. Effective, safe and easy to use treatments, preferably active in both Stage 1 AND 2 disease
This platform can also provide a base for ongoing educational cooperation between the countries in the central African region, facilitating sustainable disease control activities and standardising procedures and practices.
DNDi has facilitated the creation and maintenance of this platform both financially and through general administrative and logistic support through the DNDiAfrica office in Nairobi, and the STI-DNDiAfrica representation in Kinshasa. The HAT Platform encompasses over 30 clinicians and scientists from Uganda, DRC, Republic of Congo, Sudan, Angola, DNDi, WHO, Epicentre, STI, Institute of Tropical Medicine Antwerp, Medecins Sans Frontieres (MSF), Malteser, Kari Trypanosomiasis Research Centre, Find Diagnostics, and Centers for Disease Control and Prevention (CDC).
Hat platform objectives
To bring the key players together to
• jointly assess and define the priorities in terms of capacity needs based on
• national HAT control policies and research agendas
• patients' needs
• formulate the activities that should be delivered by the network and its partners to create sustainable clinical trial capacities in the trypanosomiasis-endemic regions.
To bring the key players together to
• jointly assess and define the priorities in terms of capacity needs based on
• national HAT control policies and research agendas
• patients' needs
• formulate the activities that should be delivered by the network and its partners to create sustainable clinical trial capacities in the trypanosomiasis-endemic regions.
Published by Drugs for Neglected Diseases Initiative - 1 Place St Gervais 1201 Geneva Switzerland - Photo credits: DNDi
Editor: Ann-Marie Sevcsik - Tel: +41 22 906 9230 - Fax: +41 22 906 9231 - www.dndi.org
Editor: Ann-Marie Sevcsik - Tel: +41 22 906 9230 - Fax: +41 22 906 9231 - www.dndi.org