-
North America
In 2018, DNDi North America continued its collaboration with the Center of Excellence for Chagas Disease in Los Angeles, publishing several joint articles describing barriers to access and models of care for people with Chagas, and supported DNDi’s global efforts to increase access to diagnosis and treatment, particularly in the Americas. In addition, the office garnered significant global media coverage of the approval of fexinidazole for sleeping sickness. In October, the office held a special event in New York City in celebration of DNDi’s 15-year anniversary, which raised awareness and funds for DNDi. Targeted advocacy was also carried out in the US, Canada, and the UN to advance policies that enable needs-driven R&D and affordable treatment access.
-
Latin America
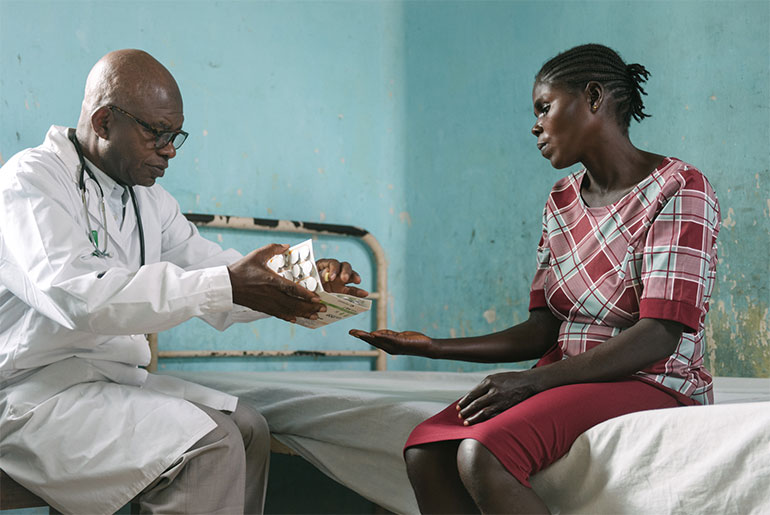
In 2018, the Latin America office completed two clinical studies for Chagas disease – one evaluating different benznidazole regimens in Bolivia and the other assessing the efficacy of fexinidazole – and completed patient enrollment in a clinical study on cutaneous leishmaniasis in Colombia and Peru. New projects were launched to increase access to Chagas diagnosis and treatment in Guatemala and Brazil, following the success of pilot projects in Colombia. DNDi also announced a collaboration with Insud Pharma and Pharco to increase access to affordable hepatitis C treatment in Latin America. The office also established official relations with the Pan-American Health Organization to strengthen collaborative efforts on these diseases as well as on innovation and access policies in the region and supported GARDP’s global observational study of newborns with sepsis.
-
East Africa
In 2018, the Africa office supported implementation of clinical trials in leishmaniasis – with two new trials starting for shorter and simpler treatment for VL and for PKDL – as well as ongoing trials for mycetoma and HIV. The office also supported GARDP’s first clinical trial on the use of the antibiotic fosfomycin in newborns with sepsis. In October, more than 400 participants from 150 institutions in 40 countries joined DNDi for its 11th Partners Meeting in Kampala, Uganda on collaboration and R&D innovation. The Nairobi office also organized a Health Science Journalism Workshop for 18 journalists from 11 African countries, a first for DNDi in our objective to raise the profile of neglected patients’ needs. In addition, the office upgraded its quality standards ISO certification to the new ISO 9001:2015.
-
Democratic Republic of Congo
The highlight for 2018 for the Kinshasa project office was the approval in the DRC of fexinidazole, the first oral treatment for sleeping sickness, a mere 39 days after the drug received a positive scientific opinion from the European Medicines Agency. The office also supported ongoing clinical studies in the DRC and Guinea testing both fexinidazole and acoziborole, as well as steering the Human African Trypanosomiasis Platform - EANETT Joint Scientific meeting in Uganda.
-
Southern Africa
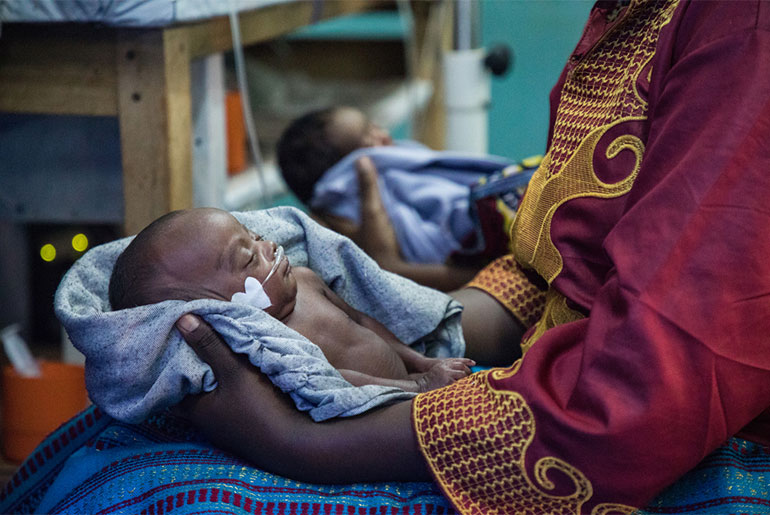
The joint DNDi-GARDP office continued to strengthen its collaborative efforts with the South African Medical Research Council (SAMRC). SAMRC provided 4 million Rand (~240,000 EUR) to further GARDP’s activities in South Africa to deliver affordable, new or improved antibiotic treatments for drug-resistant bacterial infections, beginning with neonatal sepsis and sexually-transmitted infections. GARDP launched a global observational study of babies with sepsis, with three South African sites, to guide development of new and improved antibiotic treatments for newborns. Recruitment of a team to provide support to the launch and execution of clinical trials in South Africa was also completed.
-
South Asia
DNDi in India continues to support the Indian National Kala-azar Elimination Programme in building capacity to diagnose VL and PKDL, and by gathering evidence needed to respond to threats to the sustainable elimination of VL in the region. In 2018, DNDi in India partnered with MSF on a study for better treatment of HIV/VL co-infection in Bihar and completed recruitment for a clinical trial for shorter and safer PKDL treatments. The office also hosted the launch of GARDP’s global observational study to collect clinical information on treatment of babies with sepsis, to guide the development of new and improved antibiotic treatments for newborns.
-
South-East Asia
DNDi’s Malaysia office supports the Malaysian Ministry of Health to enhance the country’s public health approach to hepatitis C and in 2018 partnered with the Foundation for Innovative New Diagnostics (FIND) to support the national plan to decentralize hepatitis C testing and treatment. The office has played a key role in DNDi’s Phase II/III clinical trial evaluating the effectiveness of a ravidasvir/ sofusbuvir combination to treat hepatitis C and presented the positive interim results to national stakeholders in Malaysia and Thailand in 2018. In addition, the office supported GARDP’s global observational study of newborns with sepsis.
-
Japan
In 2018, key partnerships were secured with Japanese pharmaceutical partners: Astellas and Daiichi Sankyo RD Novare joined ongoing or new DNDi drug discovery projects, with support from Japan’s GHIT Fund, and a new screening project to discover potential compounds against antimicrobial-resistant bacteria was launched with Eisai and Takeda. Ongoing partnerships saw progress: a new drug candidate for VL, developed by Takeda with GHIT support, advanced into pre-clinical development. And DNDi’s Tokyo office supported the launch of JAGntd, a Japanese NTD network to promote Japan’s contribution to the global effort to control and eliminate NTDs.