- More effective treatment for children co-infected with HIV and tuberculosis (TB)
- “Super-boosting” the dose of ritonavir, an antiretroviral used to treat HIV in children, counteracts negative interactions between common TB drug rifampicin and HIV therapy
- WHO revised its guidelines to recommend “superboosting” of ritonavir when treating co-infected children (2016)
Responding to
Neglected Patients’ Needs
Through Innovation
2017 in numbers
666,917
PEOPLE
screened for sleeping sickness by 10 mobile teams in DR Congo and treated for a variety of diseases
OVER
2,500
PATIENTS
enrolled in the clinical studies completed in 2017
685
PEOPLE
trained to support clinical research, from Good Clinical Practice to the use of new diagnostic technologies and processes
44
R&D PROJECTS UNDERWAY
covering every stage of the R&D process
12
new R&D partnerships
869
PEOPLE
working on DND
170,407
COMPOUNDS SCREENED
to look for promising “hits” as potential drug candidates
3+2
3 COMPOUNDS advanced to pre- clinical stage, and 2 CANDIDATES advanced to clinical development
21
CLINICAL TRIALS
in progress in 7 disease areas at 52 sites in 15 countries
2
TECHNOLOGY TRANSFERS
in Malaysia and in Latin America – for the manufacture and supply of hepatitis C treatments
€ 6
MILLION
Value of in-kind contributions from partners
DNDi R&D portfolio
December 2017
Key R&D achievements in 2017
CLICK ON THE ICONS TO DISCOVER MORE
-
RESEARCH
-
TRANSLATION
-
DEVELOPMENT
-
IMPLEMENTATION
2017 achievements
from DNDi regional offices
TAP ON PINS TO DISCOVER MORE
- Strategic leadership and coordination of research and development, from drug discovery through clinical development and registration
- Administrative and support departments, including finance, human resources, IT, policy advocacy, communications, and fundraising
- About half of all DND
i staff - Re-organization process started in 2017 to ensure structure keeps pace with evolution of DND
i portfolio.
- Approval by the U.S. Food and Drug Administration (FDA) of Chemo Group’s (now InSud Pharma) benznidazole for children with Chagas disease in close collaboration with Exeltis, Mundo Sano, and DND
i - A DND
i -supported survey of almost 5,000 Latin American-born residents of Los Angeles County found that 1.24% tested positive for Chagas disease - Advocacy at the UN High-Level Political Forum on Sustainable Development in New York and continued support for global health R&D in Washington, DC in a changing political environment
- A campaign to raise funds for African sleeping sickness secures over USD 500,000 in new private contributions that will help put the disease on the path to sustainable elimination
- Completion of recruitment in the clinical study of new regimens of benznidazole monotherapy and in combination with fosravuconazole in Bolivia for the treatment of Chagas disease
- Start of a Phase II clinical study in Spain to test short courses of fexinidazole for Chagas disease
- Implementation of a new, simplified diagnostic algorithm and treatment for all patients with Chagas disease, including chronic patients, as part of the pilot Chagas Access Programme in Colombia
- Start of a clinical study on cutaneous leishmaniasis combining thermotherapy with miltefosine in Colombia and Peru
- Opening of a new DND
i -GARDP joint liaison office in Cape Town - Collaboration agreement with South African Medical Research Council
- Support completion of fexinidazole clinical studies in adults (stage 1 and 2) and children with sleeping sickness
- Start of Phase IIIb study in seven clinical sites in DR Congo to generate information on fexinidazole in special population groups in in- and out-patients
- Start of Phase II/III study in nine clinical sites in DR Congo to test acoziborole as a single-dose treatment in patients with stage 1 or 2 sleeping sickness
- Support for the first-ever clinical trial in eumycetoma, studying the efficacy of the anti-fungal drug fosravuconazole at the Mycetoma Research Centre, Sudan
- Preliminary evaluation of the paediatric HIV ‘LIVING’ study: high levels of viral suppression in infants and young children after 48 weeks of treatment
- Initiation of the first site for the miltefosine/paromomycin clinical trial in Sudan for visceral leishmaniasis (VL) in Eastern Africa
- Community meetings in Amudat, Uganda, and Kacheliba and West Pokot, Kenya to share findings of the miltefosine dosing clinical trial in children with primary VL
- Stakeholders’ meeting in Addis Ababa, Ethiopia on clinical trial for HIV/VL coinfected patients
- ISO 9001:2008 certification (quality management systems) received for Nairobi office
- After completion of patient recruitment in the sofosbuvir/ ravidasvir clinical study for hepatitis C in Malaysia, start of recruitment in Thailand
- Approval by Malaysia of a “government use” licence to secure access to more affordable treatments for hepatitis C
- Agreement signed between pharmaceutical companies Pharmaniaga (Malaysia) and Pharco Pharmaceuticals (Egypt), and DND
i to supply affordable hepatitis C treatment in Malaysia
- NTD Drug Discovery Booster presented with Japanese pharmaceutical partners at the Annual Meeting of the Pharmaceutical Society of Japan
- Official side event on NTDs at the Universal Health Coverage Forum in partnership with Uniting to Combat NTDs, Nagasaki and St Luke’s International Universities
- Renewed support of GHIT Fund to projects on leishmaniasis, Chagas disease, and eumycetoma
- Support of Indian National Kala-azar Elimination Programme in building capacity to diagnose kala-azar and post-kala-azar dermal leishmaniasis (PKDL)
- Study to determine prevalence of PKDL in previously treated leishmaniasis patients
- Technical partner in an MSF study on kala-azar-HIV co-infection in Bihar, testing treatments with AmBisome monotherapy and AmBisome-miltefosine combination
- Start of a clinical trial for PKDL, testing AmBisome monotherapy and AmBisome-miltefosine combination
- Expansion of the Open Source Network project with an additional Indian university
The WHO-DND

The Disease that Strikes Back
Could a form of leishmaniasis challenge elimination efforts in India?
READ THE STORY
7 new treatments delivered, recommended & implemented since 2007

Drug discovery and translational research
Open approaches and increased efficiency for new chemical entities
DND
NTD Drug Discovery Booster: speeding up drug discovery & cutting costs
- 2 new partners in 2017 - 8 partners total
- Millions of compounds screened for leishmaniasis and Chagas disease through a simultaneous search process across the pharmaceutical companies
- 12 hit series identified, 4 of these investigated for proof of principle

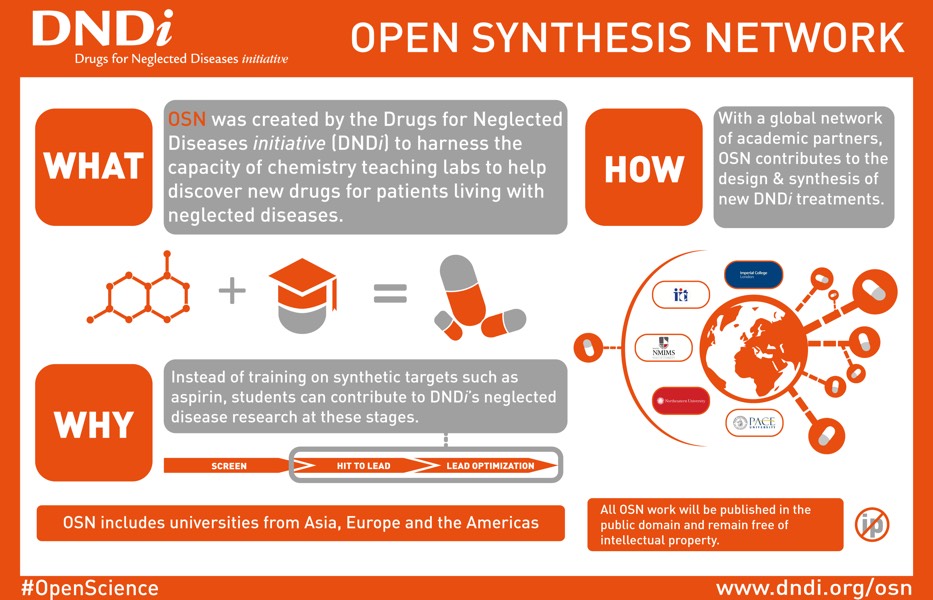
Open Synthesis Network: crowdsourcing compound synthesis
- 6 new partners in 2017 - 12 partner universities total
- Master's and undergraduate students synthesizing compounds for leishmaniasis and Chagas disease
- Partners in Brazil, India, Switzerland, UK, and USA
Unprecedented leishmaniasis portfolio: Towards new-generation treatments
- New chemical classes with different mechanisms of action against
Leishmania parasites - 6 compounds in pre-clinical development
- 2 compounds ready to enter clinical development in 2018
- 1 new combination for cutaneous leishmaniasis in Proof of concept study


Mycetoma Clinical
Trial Begins
WATCH FILM
2017 clinical activities:
21 trials, 4,000+ participants
Clinical trial data transparency
DND
DISEASES
- Human African trypanosomiasis
- Leishmaniasis
- Chagas disease
- Filarial disease
- Mycetoma
- Paediatric HIV
- Hepatitis C
Click on the diseases to see related sites
Filarial diseases
- Emodepside single ascending dose for onchocersiasis (UK)
- Safety, tolerability, and PK of multiple ascending doses of emodepside (UK)
- Relative bioavailability study of emodepside immediate release tablets and solution (UK)
HAT
- Acoziborole pivotal study in adults with stage 1 and stage 2 HAT (DR Congo)
Mycetoma
- Fosravuconazole proof-of-concept for eumycetoma patients (Sudan)
Cutaneous leishmaniasis
- Thermotherapy & miltefosine combination proof-of-concept (Colombia, Peru)
PKDL
- Short-course regimens for treatment of PKDL (Sudan)
- Short-course regimens for treatment of PKDL (India, Bangladesh)
Chagas disease
- Benznidazole new doses, improved treatment, and therapeutic associations (Bolivia)
- Fexinidazole proof-of-concept (Spain)
HAT
- Study of fexinidazole in special population groups, in in- and out-patients (DR Congo)
- Fexinidazole pivotal study (DR Congo)
- Fexinidazole study in adults with early-stage 1 + stage 2 HAT (DR Congo)
- Fexinidazole study in children with both stage 1 + 2 HAT (DR Congo)
Hepatitis C
- Ravidasvir/sofosbuvir combination therapy (Malaysia, Thailand)
Paediatric HIV
- Lopinavir/ritonavir pellets with dual NRTIs implementation study in infants and young children (Kenya, Uganda, Tanzania)
Visceral leishmaniasis
- Miltefosine/paromomycin Phase III trial for treatment of primary visceral leishmaniasis (VL) patients in Eastern Africa (Ethiopia, Kenya, Sudan, Uganda)
PKDL
- Infectivity study of PKDL patients (Bangladesh)
HIV-VL
- New treatments for HIV-VL co-infection (MSF
study sponsored by DND
i ) (India) - New treatments for HIV-VL co-infection (MSF
study sponsored by DND
i ) (Ethiopia)
PKDL
- Follow-up study of PKDL patients (India)
Clinical trial data transparency
DND
HIV: Introducing oral pellets for children living with HIV
Since 2015, DND
Sleeping sickness: A study for the Congolese run by the Congolese
More than 200 people in the Democratic Republic of the Congo
(DR Congo) have been actively engaged in DND
Hepatitis C: Enabling public health impact
DND
PHASES
- PHASE I
- PHASE II a/PoC
- PHASE IIb/III
- PHASE IV
STORIES

Challenges of Conducting Clinical Trials in Remote Areas
WATCH FILM
Strengthening capacities
Disease-specific research platforms and networks are central to DND
As we work with endemic-country partners to conduct clinical trials, central to DND
The disease-specific research platforms and networks created by DND
685 PEOPLE TRAINED IN 2017
Platforms & Networks

Chagas Clinical Research Platform (CCRP)
Founded in 2009 in Uberaba, Brazil
424 members from over 150 institutions
RedeLEISH
Founded in 2014 in Rio de Janeiro, Brazil
139 members, from 62 institutions

HAT Platform
Founded in 2005 in Kinshasa, DR Congo
120 members from over 20 institutions

Leishmaniasis East Africa Platform (LEAP)
Founded in 2014 in Rio de Janeiro, Brazil
139 members, from 62 institutions

Access - Transitioning from registration to implementation
Ending the Neglect of Chagas in Colombia
SEE STORY
Hepatitis C: A public health approach for access
Despite availability of effective and safe treatment options for hepatitis C virus (HCV), access to treatment is out of reach for millions due to high prices. DND
Results suggest that the ravidasvir/sofosbuvir combination is comparable to the best treatments available today, with a radical difference: price. But to use this drug combination, countries would also need access to affordable sofosbuvir. In September 2017, Malaysia issued a “government use” licence to source generic sofosbuvir, a move which has allowed it to accelerate access to affordable HCV treatment in its public hospitals.

Fexinidazole: Using the regulatory process to pave the way for access
In December 2017, a few months after the conclusion of large-scale DND
If fexinidazole is registered in late 2018, regulatory approval will only be the first step. Translation into national policy and treatment guidelines will be needed as well as community awareness efforts and treatment knowledge for health providers.

HIV: Preparing for the 4-in-1 by encouraging uptake of the 2-in-1
DND

Policy, fundraising & communications
6
NATIONAL TREATMENT POLICIES
or guidelines revised to reflect the use of DND
EUR
83.4
MILLION
Multi-year funds secured for DND
22
PEER-REVIEWED SCIENTIFIC PUBLICATIONS
on DND
Policy
WHO Director General Dr Tedros has declared universal health coverage (UHC) to be the priority for his mandate. This has important implications for NTDs, which are diseases of poverty affecting most countries. Delivering quality UHC depends on the availability of and access to safe, effective, and affordable medicines, underscoring the continued need for R&D for NTDs as a key component of the UHC agenda. NTD programmes provide entry points to some of the world’s hardest-to-reach communities, and many policymakers have identified the ability to address NTDs as a “litmus test” for UHC.
The Berlin Declaration that followed the first-ever G20 Health Ministers meeting in May 2017 cautioned that success in the fight against AMR cannot be achieved with current treatments. It recognized the importance of ‘reactivating the R&D pipeline through incentive mechanisms that avoid the reliance on high price/volume combinations’ to ensure sustainable access. It called for ‘broadening the voluntary financial support’ for initiatives, including GARDP, which ‘reinvigorate R&D in science and industry for antimicrobials’.
2017 was marked by the Malaysian government’s decision to reaffirm its strong commitment to providing access to treatments for hepatitis C by issuing a “government use” licence enabling access to more affordable versions of sofosbuvir, an expensive and patented treatment for hepatitis C. This is a landmark decision for the more than 400,000 people living with hepatitis C in Malaysia.
Fundraising
Funds raised in 2017 for the neglected disease portfolio include increased support for the sleeping sickness programme from the Bill & Melinda Gates Foundation, as well as from private donors primarily from the US, through the HAT campaign launched last year, but also in Europe.
Other noteworthy contributions include the first grant from
the GHIT Fund secured for mycetoma, and from the European and Developing Countries Clinical Trials Partnership for a visceral leishmaniasis clinical trial in four African countries. In addition, the first grant entirely dedicated to DND
In May, the G20 Health Ministers cautioned that success in the fight against antimicrobial resistance (AMR) cannot be achieved with the current treatments. The Declaration welcomed and sought to build on initiatives such as GARDP, to ‘reinvigorate research and development in science and industry for antimicrobials.’ The German Federal Ministry of Health and Ministry of Education and Research then hosted a pledging conference for GARDP in September 2017. A total of EUR 56 M was pledged by Germany, Luxembourg, Monaco, the Netherlands, South Africa, Switzerland, the United Kingdom, and the Wellcome Trust for the development of new and improved treatments to fight antibiotic resistance and contribute to ensuring healthy lives and well-being for all. Of this total, EUR 5.5 M was received in 2017.
Since 2003, DND
Communications
In 2017, DND
In 2017, DND
In recognition of its innovative and collaborative model, DND
A word of thanks
DND
Photo credits: Benoit Marquet-DNDi; Kishore Pandit-DNDi; Felipe Abondano-DNDi; Neil Brandvold-DNDi; Bobby Tan-DNDi; Scholars and Gentlemen/DNDi.












